Oxidative Stress
Oxidative stress is a condition characterized by a change in the balance between pro-oxidants and anti-oxidants in favor of the pro-oxidant state- this increases production of reactive oxygen species (ROS), or free radicals.
Oxygen (O2) is essential to life. Human cells and cells of other aerobic organisms require molecular oxygen (O2) in order to survive and produce energy (ATP). The energy-producing “factories” of our cells are called mitochondria. During the process of metabolism and utilization of molecular oxygen (O2) by mitochondria for energy production, single electrons may escape. They react with oxygen and generate oxygen-free radicals such as superoxide (singlet oxygen) radicals (O2-), hydroxy radicals (OH–), and peroxide such as hydrogen peroxide (H2O2). They are highly reactive free radicals (also called reactive oxygen species or ROS) and are able to cause severe damage to cell components such as DNA, RNA, proteins, and phospholipids. They are like cellular atomic bombs. Free radical formation increases with increasing metabolic activity, and are also generated from exposure to external factors such as smoking, medication, alcohol, radiation, toxins, and xenobiotics.
It seems counterintuitive that oxygen, an element we depend on for survival and consider a “best friend”, can cause such harm. Without oxygen we cannot survive, while at the same time, oxygen free radicals are the main contributor to degenerative diseases of aging.
Lipid hydroperoxides are formed from the action of reactive oxygen species on membrane phospholipids (polyunsaturated fatty acids). Lipid peroxidation causes cell damage and is an indicator of oxidative stress damage. Increased levels of lipid peroxidation are associated with various conditions such as cancer, multiple sclerosis, aging, intense exercise, cardiovascular disease and neurological conditions. Lipid peroxides are unstable and break down in different compounds, the most abundant of which is malondialdehyde (MDA). Malondialdehyde is an end-product of peroxidation of polyunsaturated fatty acids (PUFA) and its measurement is an indicator of lipid peroxidation, and an elevated level of oxidative damage. Malondialdehyde (MDA) is highly toxic, reacting with and damaging DNA, phospholipids, amino acids and protein. Higher levels of MDA are found as we age, and its measurement has been suggested as a possible marker of aging.
Our body tries to protect itself against free radicals using antioxidants. There are different types of antioxidants:
- Antioxidants which help to prevent the formation of free radicals such as albumin ceruloplasmin, ferritin, metallothionine and transferrin.
- Enzymes such as superoxide dismutase (SOD), mitochondrial SOD (MnSOD), and cytoplasmatic SOD (CuZnSOD), catalase, and glutathione peroxidase.
- Others such as bilirubin, carotenoids, ascorbate, glutathione, flavenoids, uric acid and melatonin
Some antioxidant are water-soluble, such as ascorbic acid and glutathione. Other antioxidants are lipid-soluble, such as vitamin A and tocopherols. Some are synthesized in the body, other depend on dietary intake, such as ascorbic acid. Ceruloplasmin and tranferrin are protein antioxidants.
Superoxide dismutase is an essential antioxidant enzyme that converts singlet oxygen to hydrogen peroxide (H2O2)
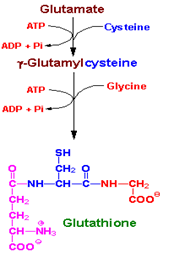
Glutathione is a tripeptide made from three amino acids- glutamic acid, cysteine and glycine. Glutathione exists in two forms, reduced glutathione (GSH), and the oxidized glutathione (GSSG) also known as glutathione disulfide. Inside cells, we primarily find the reduced form of glutathione (GSH) which is an antioxidant rich in electrons. It is an essential co-factor for the antioxidant enzyme glutathione peroxidase. It is also involved in transferring glutamine to other amino acids and in various detoxification pathways, binding to heavy metals and pesticides to bring them out of the body. Reduced glutathione is able to conserve vitamin C, and vice versa.
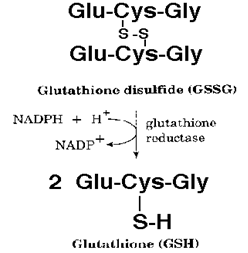
Glutathione peroxidase is an important enzyme needed for intracellular antioxidant defences. It plays an essential role in human defence against oxidative damage. This is done by breaking down hydrogen peroxide and other hydroperoxides (such as lipid hydroperoxide, and DNA hydroperoxide) into water, with secondary oxidation of the reduced glutathione (GSH) to oxidized glutathione (GS-SG).
H2O2 –> H2O (water) + O + Reduced Glutathione = H2O (water) + Oxidized Glutathione
A low level of glutathione peroxidase is an indicator of oxidative stress and has been associated with degenerative diseases of aging such as cancer. Glutathione peroxidase contains selenium. Selenium is an essential trace element whose deficiency may affect the activity of glutathione peroxidase. At normal levels, selenium may be protective against diseases but at high concentrations it is toxic. It is important to monitor selenium levels in order to keep them at a normal range.
Oxidative Stress results from a disruption of the balance between levels of pro-oxidant and anti-oxidants. Many diseases have been associated with low antioxidant levels. Measuring selenium, malondialdehyde, glutathione peroxidase and monitoring free radical status is essential for cell health.
References:
Ozden M, et al. Erythrocyte glutathione peroxidase activity, plasma malondialdehyde and erythrocyte glutathione levels in hemodialysis and CAPD patients. Clin Biochem. 2002 Jun;35(4):269-73.
Deng H, Zhou H, Sun M. Roles of sex hormones and oxygen free radical in coronary heart disease. Hunan Yi Ke Da Xue Xue Bao. 1999;24(4):343-6.
Haulica I, Boisteanu P, Iliescu R. Implications of oxidative stress in senescence. Rev Med Chir Soc Med Nat Iasi. 2000 Apr-Jun;104(2):15-9.
Kumaraguruparan R, Subapriya R, Viswanathan P, Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2002 Nov;325(1-2):165.
Gil P, Farinas F, Casado A, Lopez-Fernandez E. Malondialdehyde: a possible marker of ageing. Gerontology. 2002 Jul-Aug;48(4):209-14.
Keles MS, Taysi S, Sen N, Aksoy H, Akcay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can J Neurol Sci. 2001 May;28(2):141-3.
Guerra A, et al. Lipid profile and redox status in high performance rhythmic female teenagers gymnasts. J Sports Med Phys Fitness. 2001 Dec;41(4):505-12.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21.
Sewerynek E, Reiter RJ, Melchiorri D, Ortiz GG, Lewinski A. Oxidative damage in the liver induced by ischemia-reperfusion: protection by melatonin. Hepatogastroenterology. 1996 Jul-Aug;43(10):898-905.
Melchiorri D, Reiter RJ, Chen LD, Sewerynek E, Nistico G. Melatonin affords protection against kainate-induced in vitro lipid peroxidation in brain. Eur J Pharmacol. 1996 Jun 3;305(1-3):239-42.
Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999 Nov;27(9-10):951-65.
Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood). 2002 Oct;227(9):671-82.
Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann Intern Med 1987;107:526-545
Sacheck JM. Blumberg JB. Role of vitamin E and oxidative stress in exercise. Nutrition. 17(10):809-14, 2001 Oct.
Blair IA. Lipid hydroperoxide-mediated DNA damage. Experimental Gerontology. 36(9):1473-81, 2001 Sep.
Browne SE. Ferrante RJ. Beal MF. Oxidative stress in Huntington’s disease. Brain Pathology. 9(1):147-63, 1999 Jan.
Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radical Biology & Medicine. 28(12):1685-96, 2000 Jun 15.
Slatter DA. Bolton CH. Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 43(5):550-7, 2000 May.
Rimbach G. Hohler D. Fischer A. Roy S. Virgili F. Pallauf J. Packer L. Methods to assess free radicals and oxidative stress in biological systems. Archiv fur Tierernahrung. 52(3):203-22, 1999.
Moore K. Roberts LJ. Measurement of lipid peroxidation. Free Radical Research. 28(6):659-71, 1998 Jun.
